
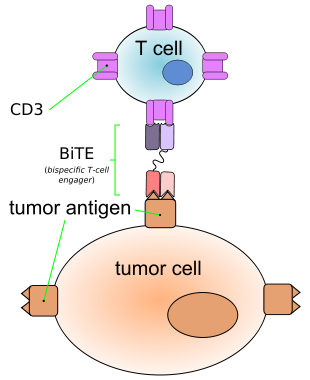
September 24, Anjin Xuan cloth FDA has submitted its Bi-specific T-cell engager (BiTE) antibody drug blinatumomab biologics license application (BLA), seeking approval for the Philadelphia chromosome negative (Ph-) recurrent / hard treatment of acute B lymphocyte precursor cells to treat leukemia (ALL) in. The drug is a single-chain bispecific T cell antibody (BiTE), simply, it uses an antibody killer T cells can be re-directed to destroy tumor cells.
The drug not only by the FDA and EMA have granted orphan drug status, FDA also granted the drug a breakthrough therapy identified.
December 21, 2012, the American magazine "Science" will also be included in cancer immunotherapy 2013, one of six most noteworthy scientific fields.
Magazine "Science" that the scientific community has recently been developed that can drive the body's immune cells to fight cancer drugs, and to help a small portion of patients affected by cancer to overcome the disease plagued.
Researchers predict that the two targeted immunotherapies for the combination of different biological pathways, will be more intense blow to cancer cells.
The main reason for immunotherapy is currently being sought traditional treatment (mainly radiotherapy and chemotherapy and surgery, etc.) compared to tumor immunotherapy selective killing effect on tumor stronger and toxicity smaller advantage.
From biological exploration

